Introduction:Chronic myeloid leukemia (CML) is a myeloproliferative disorder characterized by the unregulated proliferation of myeloid cells in the bone marrow and their accumulation in the blood. The approval of imatinib (IM) and second-generation TKIs have substantially improved survival for patients with CML. Despite the effectiveness of these TKIs in controlling CML, continuous therapy is necessary for most patients, and may be associated with the development of unfavorable adverse events such as hepatotoxicity. Although patients with elevated liver function tests are excluded from key clinical trials, case reports and small single-center studies have described cases of TKI-associated hepatotoxicity among patients with CML. This study sought to describe the prevalence and incidence of hepatotoxicity in patients with newly diagnosed Philadelphia chromosome-positive chronic-phase (Ph+ CP) CML treated with 1L TKIs in community oncology practices.
Methods:This retrospective, observational cohort study identified adult patients newly diagnosed with Ph+ CP CML and treated with 1L dasatinib (DAS), IM, or nilotinib (NIL) within the US Oncology Network (USON) between 1 January 2008 and 30 November 2018. Patients were required to have at least one comprehensive metabolic panel (CMP) within 90 days of treatment start, and ≥ 1 CMP during follow-up. Additionally, patients had to have at least two visits within the USON, no enrollment in any clinical trial during the study period, and no documentation of other primary cancer diagnoses. Descriptive statistics were generated for baseline patient characteristics and prevalence and incidence of hepatotoxicity, and were stratified by 1L TKI treatment initiation. Grades for hepatotoxicity were derived from laboratory data using the Common Terminology Criteria for Adverse Events version 5.
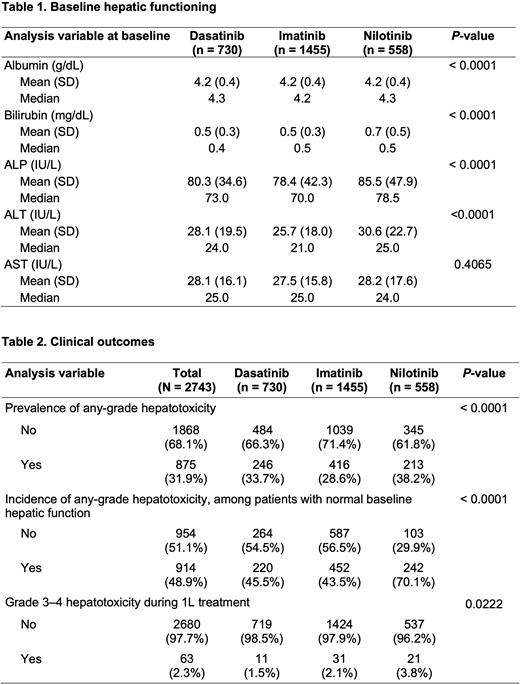
Results:A total of 2743 patients with CP-CML were identified. Of those, 730 (26.6%), 1455 (53.0%), and 558 (20.3%) patients were treated with 1L DAS, IM, or NIL, respectively. With the exception of age and region, patient demographic characteristics were well balanced across cohorts. However, baseline hepatic functioning differed across TKIs (Table 1). Prevalence of any-grade liver enzyme elevation was observed in almost one-third (31.9%) of patients, including 246 (33.7%) patients treated with DAS, 416 (28.6%) patients treated with IM, and 213 (38.2%) patients treated with NIL. Among all patients with normal baseline hepatic function, 48.9% developed any-grade hepatotoxicity while on 1L therapy. Across TKI cohorts, significantly more patients treated with NIL developed any-grade hepatotoxicity (70.1%) compared to patients treated with DAS (45.5%) or IM (43.5%) (P< 0.0001). Among all patients receiving 1L treatment, 1.5% to 3.8% of patients experienced grade 3-4 hepatotoxicity. Significant differences in the proportion of patients that developed grade 3-4 hepatotoxicity were observed across treatments and were most common among patients treated with NIL (Table 2).
Conclusions:Findings from this analysis suggest that hepatic dysfunction may be common at baseline among patients with CP-CML treated in real-world community oncology settings. Among patients with normal baseline hepatic function, more patients treated with NIL experienced any-grade hepatotoxicity. Patients treated with NIL were also more likely to develop grade 3-4 hepatotoxicity compared to patients treated with DAS or IM. These findings may provide insight into the effects of long-term TKI treatment on hepatic functioning and help to inform treatment choices for patients.
Kolibaba:AbbVie:Research Funding;Compass Oncology:Ended employment in the past 24 months;Janssen:Research Funding;TG Therapeutics:Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding;McKesson Life Sciences:Consultancy;Sumitomo Dainippon Pharma Oncology:Consultancy, Other: travel, accommodations, expenses, ;Gilead:Research Funding;Genentech:Research Funding;Cell Therapeutics:Research Funding;Celgene:Research Funding;Acerta:Research Funding;Atara Biotech:Membership on an entity's Board of Directors or advisory committees;Verastem:Honoraria;Seattle Genetics:Research Funding;Novartis:Research Funding;Pharmacyclics:Research Funding.Zhou:McKesson Corporation:Current Employment.Keating:Bristol Myers Squibb:Current Employment.Clark:McKesson Life Sciences:Current Employment, Current equity holder in publicly-traded company.Brokars:Bristol Myers Squibb:Current Employment.Kee:Bristol Myers Squibb:Current Employment.Copher:Bristol Myers Squibb:Current Employment.Stwalley:Bristol Myers Squibb:Current Employment, Current equity holder in publicly-traded company.Jabbour:Adaptive Biotechnologies:Other: Advisory role, Research Funding;Genentech:Other: Advisory role, Research Funding;BMS:Other: Advisory role, Research Funding;Amgen:Other: Advisory role, Research Funding;Pfizer:Other: Advisory role, Research Funding;Takeda:Other: Advisory role, Research Funding;AbbVie:Other: Advisory role, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal